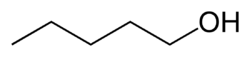
1-Pentanol
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentan-1-ol[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 1730975 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.684 |
| EC Number |
|
| 25922 | |
| KEGG | |
| MeSH | n-Pentanol |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1105 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.150 g·mol−1 |
| Density | 0.811 g cm−3 |
| Melting point | −78 °C; −109 °F; 195 K |
| Boiling point | 137 to 139 °C; 278 to 282 °F; 410 to 412 K |
| 22 g L−1 | |
| log P | 1.348 |
| Vapor pressure | 200 Pa (at 20 °C) |
| -67.7·10−6 cm3/mol | |
Refractive index (nD)
|
1.409 |
| Thermochemistry | |
Heat capacity (C)
|
207.45 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
258.9 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−351.90–−351.34 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−3331.19–−3330.63 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H226, H315, H332, H335 | |
| P261 | |
| NFPA 704 (fire diamond) | |
| Flash point | 49 °C (120 °F; 322 K) |
| 300 °C (572 °F; 573 K) | |
| Related compounds | |
Related compounds
|
Hexane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Pentanol, (or n-pentanol, pentan-1-ol), is an organic compound with the formula CH3CH2CH2CH2CH2OH and is classified as a primary alcohol.[2] It is a colourless liquid with a distinctive aroma. It is one of 8 isomeric alcohols with the formula C5H11OH. It is used as a solvent, a biological drying agent and in the synthesis of some fragrance compounds. It is also a common component of fusel alcohols (fusel oils), the undesirable byproducts of alcoholic fermentation.
Preparation
[edit]1-Pentanol is prepared from 1-butene by hydroformylation followed by hydrogenation of the resulting pentanal.[3]
- CH3CH2CH=CH2 + CO + H2 → CH3CH2CH2CH2CHO
- CH3CH2CH2CH2CHO + H2 → CH3CH2CH2CH2CH2OH
Pentanol can be prepared by fractional distillation of fusel oil. To reduce the use of fossil fuels, research is underway to develop cost-effective methods of producing (chemically identical) bio-pentanol with fermentation.[4][5]
Uses and occurrence
[edit]The hydroxyl group (OH) is the active site of many reactions. The ester formed from 1-pentanol and butyric acid is pentyl butyrate, which has an apricot-like odor. The ester formed from 1-pentanol and acetic acid is amyl acetate (also called pentyl acetate), which has a banana-like odor.
It is a precursor to dipentyl zinc dithiophosphates, which are used in froth flotation.[3]
In 2014, a study was conducted comparing the performance of diesel fuel blends with various proportions of pentanol as an additive. While gaseous emissions increased with higher concentrations of pentanol, particulate emissions decreased.[6]
Pentanol is often used as a solvent.
References
[edit]- ^ "n-pentanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 10 October 2011.
- ^ CRC Handbook of Chemistry and Physics 65th ed.
- ^ a b Lappe, Peter; Hofmann, Thomas (2011). "Pentanols". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_049.pub2. ISBN 9783527303854.
- ^ Cann, Anthony F.; Liao, James C. (2010-01-01). "Pentanol isomer synthesis in engineered microorganisms". Applied Microbiology and Biotechnology. 85 (4): 893–899. doi:10.1007/s00253-009-2262-7. ISSN 1432-0614. PMC 2804790. PMID 19859707.
- ^ Tseng, Hsien-Chung (2011). Production of pentanol in metabolically engineered Escherichia coli (Thesis thesis). Massachusetts Institute of Technology. hdl:1721.1/65767.
- ^ Wei, Liangjie & Cheung, C.s & Huang, Zuohua. (2014). Effect of n-pentanol addition on the combustion, performance and emission characteristics of a direct-injection diesel engine. Energy. 70. 10.1016/j.energy.2014.03.106.

